Errors of immunity for the community allergist: identification and investigation
DOI:
https://doi.org/10.58931/cait.2022.2233Abstract
Investigation of immune function is essential for accurate diagnoses in patients with recurrent and/or unusual infections as well as those with features of immune dysregulation. Many new diagnostic tools have been added to our medical armamentarium in recent years yet the diagnosis of IEI still relies on the combination of clinical acumen to identify patients at risk, leading to appropriate laboratory and genetic tests. The early evaluation of immune function provides not only critical diagnostic information, but also guides clinical decisions regarding appropriate therapies and prevention of disease-associated morbidity and mortality. As illustrated in this article and by the clinical vignette, infection may not be the significant presenting feature for IEI. Patients for whom there are clinical suspicions for IEI should be evaluated with screening tests followed by directed protein/cellular and genetic testing. As this remains an evolving field, patients may need to be re-evaluated as our understanding progresses.
References
Bonilla FA et al., Joint task force on practice parameters, representing the American Academy of allergy, asthma & immunology; the American College of Allergy, Asthma & Immunology; and the Joint Council of Allergy, Asthma & Immunology. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136(5):1186–205.
Tangye S et al. Human Inborn Errors of Immunity: 2019 Update on the Classification from the International Union o Immunological Societies Expert Committee. J Clin Immunol. 2020; 40(1): 24–64.
Picard C et al. International union of immunological Societies: 2017 primary immunodeficiency diseases committee report on inborn errors of immunity. J Clin Immunol 2018;38(1):96e128.
Bousfiha A et al. The 2017 IUIS phenotypic classification for Primary Immunodeficiencies. J Clin Immunol. 2018;38(1):129–43.
Chi CY et al. Clinical manifestations, course, and outcome of patients with neutralizing anti-interferon-γ autoantibodies and disseminated nontuberculous mycobacterial infections. Medicine. 2016;95(25):e3927.
Notarangelo LD. Primary immunodeficiencies. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S182–94.
Zhang Q et al. Pathogenesis of infections in HIV-infected individuals: insights from primary immunodeficiencies. Curr Opin Immunol. 2017;48:122–33.
Latiff AH. The clinical significance of immunoglobulin A deficiency. Ann Clin Biochem 2007;44 (Pt 2):131e9.
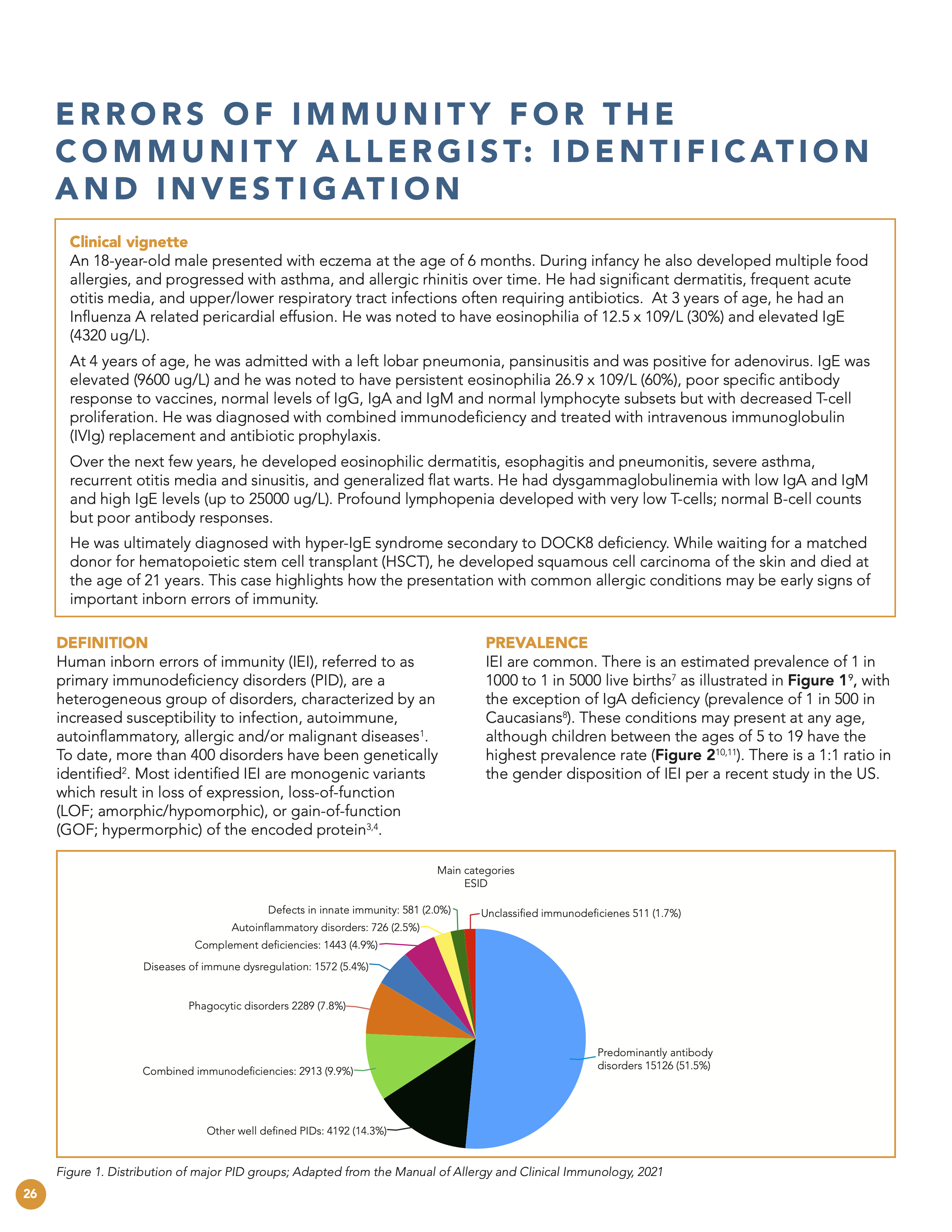
Feteih A et al. General approach Primary Immunodeficiency (PID) in adults. The Manual of Allergy and Clinical Immunology, 2021. Fig. 22.1 Distribution of the major PID groups: Percentage of diagnosed patients registered in the European Society for Immunodeficiencies (ESID database).
Bousfiha A et al. Primary immunodeficiency diseases worldwide: more common than generally thought. J Clin Immunol 2013;33(1):1e7.
Modell V, Orange JS, Quinn J, Modell F. Global report on primary immunodeficiencies: 2018 update from the Jeffrey Modell Centers Network on disease classification, regional trends, treatment modalities, and physician reported outcomes. Immunol Res 2018;66(3):367e80.
Joshi AY et al. Incidence and temporal trends of primary immunodeficiency: a population-based cohort study. Mayo Clinic proceedings Mayo Clinic 2009;84(1):16e22.
Total number of patients in the USIDnet registry through 2019. US Immunodeficiency Network 2018.
Seidel MG et al. The European society for immunodeficiencies (ESID) registry working definitions for the clinical diagnosis of inborn errors of immunity. J Allergy Clin Immunol Pract 2019;7(6):1763e70
Barbouche MR et al. Primary immunodeficiencies in highly consanguineous North African populations. Ann N Y Acad Sci 2011; 1238:42e52.
Abolhassani H et al. Fourth update on the Iranian national registry of primary immunodeficiencies: integration of molecular diagnosis. J Clin Immunol 2018;38(7):816e32.
Rezaei et al. Common presentations and diagnostic approaches. Stiehm’s Immune Deficiencies, Inborn Errors of Immunity, Elsevier. 2020;3-42.
Chinen J et al. Secondary immunodeficiencies, including HIV infection. J Allergy Clin Immunol. 2010 February ; 125(2 Suppl 2): S195–S203. doi:10.1016/j.jaci.2009.08.040.
Duraisingham S et al. Primary vs. secondary antibody deficiency: clinical features and infection outcomes of immunoglobulin replacement. PLoS ONE. 2014;9(6):e100324.
Mak T.W. et al. The Immune Response: Basic and Clinical Principles. Burlington: Elsevier/Academic Press, 2006.
Murphy K et al. Janeway’s immunobiology. WW Norton & Company, 9th edition. 2017.
Mbongue J et al. The role of dendritic cells in tissue-specific autoimmunity. J Immunol Res. 2014;2014:857143. doi: 10.1155/2014/857143.
Bousfiha A et al. Human Inborn Errors of Immunity: 2019 Update of the IUIS Phenotypical Classification. J Clin Immunol. 2020; 40(1): 66–81.
Chan A et al. Primary immune regulatory disorders: a growing universe of immune dysregulation. Curr Opin Allergy Clin Immunol. 2020 Dec;20(6):582-590.
Tangye S et al. The Ever-Increasing Array of Novel Inborn Errors of Immunity: an Interim Update by the IUIS Committee. J Clin Immunol. 2021 Apr;41(3):666-679.
Chan A et al. Hematopoietic Cell Transplantation in Patients With Primary Immune Regulatory Disorders (PIRD): A Primary Immune Deficiency Treatment Consortium (PIDTC) Survey. Front Immunol. 2020;11:239.
Cepika AM et al. Tregopathies: Monogenic diseases resulting in regulatory T-cell deficiency. J Allergy Clin Immunol. 2018 12 1;142(6):1679–95.
Chan SK et al. Primary immunodeficiency masquerading as allergic disease. Immunol Allergy Clin. 2015;35(4):767–778.
Lyons JJ et al. Primary atopic disorders. J Exp Med. 2018;215(4):1009–1022.
Milner JD. Primary atopic disorders. Annu Rev Immunol. 2020;38:785–808.
Castagnoli et al. World Allergy Organization Journal (2021) 14:100513.
Shehata N et al. The use of immunoglobulin therapy for patients with primary immune deficiency: an evidence-based practice guideline. Transfus Med Rev. 2010;24(Suppl1):S28–50.
Smith T et al. Primary B-cell immunodeficiencies. Hum Immunol 2019;80(6):351e62.
Conley ME et al. Primary B cell immunodeficiencies: comparisons and contrasts. Annu Rev Immunol 2009;27:199e227.
Orange JS et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the basic and clinical immunology interest section of the American academy of allergy, asthma & immunology. J Allergy Clin Immunol 2012;130(3 Suppl.l):S1e24.
Villa A et al. Omenn syndrome: inflammation in leaky severe combined immunodeficiency. J Allergy Clin Immunol 2008;122(6):1082e6.
Fleisher TA et al. Functional and molecular evaluation of lymphocytes. J Allergy Clin Immunol 2004;114(2):227e34.
Delmonte OM et al. Flow cytometry: surface markers and beyond. J Allergy Clin Immunol 2019;143(2):528e37.
Sullivan KE. Chromosome 22q11.2 deletion syndrome: DiGeorge syndrome/Velocardiofacial syndrome. Immunol Allergy Clin N Am 2008;28(2):353e66.
Prussin C et al. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods 1995;188(1):117-28.
Van Zelm MC et al. PID comes full circle: applications of V(D)J recombination excision circles in research, diagnostics and newborn screening of primary immunodeficiency disorders. Front Immunol 2011;2:12.
Rosenzweig S et al. Laboratory evaluation of primary immunodeficiency disorders. Stiehm’s Immune Deficiencies, Inborn Errors of Immunity, 2020; 115-130.
Knight V. et al. A Toolkit and Framework for Optimal Laboratory Evaluation of Individuals with Suspected Primary Immunodeficiency. J Allergy Clin Immunol Pract. 2021; 9:3293-307
Knutsen AP et al. Interpreting low T-cell receptor excision circles in newborns with DiGeorge anomaly: importance of assessing naïve T-cell markers. J Allergy Clin Immunol 2011;128(6):1375e6.
Rechavi E et al. Timely and spatially regulated maturation of B and T cell repertoire during human fetal development. Sci Transl Med 2015;7(276):276ra25.
Ram S et al. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev 2010;23:740-80.
Dadfar, E. et al. Fatal pneumococcus meningitis in a child with complement factor ficolin-3 deficiency. J Allergy Clin Immunol Pract. 2020 Feb;8(2):778-779
Boztug K et al. Congenital neutropenia syndromes. Immunol Allergy Clin N Am 2008;28(2):259e75.
Etzioni A et al. Leukocyte adhesion deficiencies: molecular basis, clinical findings, and therapeutic options. Adv Exp Med Biol 2007;601:51e60.
Holland SM. Chronic granulomatous disease. Hematol Oncol Clin N Am 2013;27(1):89e99.
Jirapongsananuruk O et al. Diagnostic paradigm for evaluation of male patients with chronic granulomatous disease, based on the dihydrorhodamine 123 assay. J Allergy Clin Immunol 2003;111(2):374e9.
Mace EM et al. Emerging insights into human health and NK cell biology from the study of NK cell deficiencies. Immunol Rev 2019;287(1):202e25.
Freud AG et al. Human natural killer cell development. Immunol Rev 2006;214:56e72.
Kogawa K et al. Perforin expression in cytotoxic lymphocytes from patients with hemophagocytic lymphohistiocytosis and their family members. Blood 2002;99(1):61e6.
Aydin SE et al. Hematopoietic stem cell transplantation as treatment for patients with DOCK8 deficiency. J Allergy Clin Immunol Pract. 2019;7:848–855.
Bruton OC. Agammaglobulinemia. Pediatrics 1952;9(6):722e8.
Okano T et al. Whole-Exome Sequencing-Based Approach for Germline Mutations in Patients with Inborn Errors of Immunity. J Clin Immunol. 2020 Jul;40(5):729-740.